HISTORY
One of the important parameters of water quality is the pH.1 A pH measurement reveals whether the solution is acidic, neutral, or alkaline. The chemistry of water has been discussed previously, which will help us understand what pH really is.
In the most basic sense, pH refers to the negative logarithmic (log) measurement of the H+ion concentration in solution.2 The more H+, the more acidic; the less H+, the more alkaline. The term pH was first used in 1920, but the concept was invented by Danish chemist, Soren Peter Sorenson, in 1909 to refer to negative log (inverse of an exponent) of the hydrogen ion concentration.3
The ‘p’ refers to the German word ‘potenz’ or power (power having reference to it being an exponent).4 The power referred to is the power of 10 used as the base of the log and not to the strength of the acid in solution.5
THE H+ ION
The H+ ion comes from the self-ionization or auto-proteolysis of water,6 wherein H2O splits to form H+ ion (proton) and OH– ion (hydroxide). That is H2O ? H+ + OH– However, the H+ ion is attracted to the negatively charged oxygen of another water molecule to form H3O+ ion (hydronium ion).7 Actually H+ ions don’t exist in water and the use of H+ really has reference to the hydronium ion, which is further complexed by additional water molecules.8
SELF-IONIZATION OF WATER
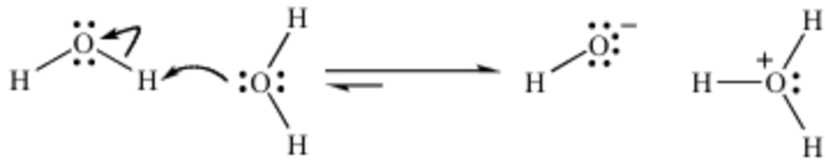

The following diagram depicts a more accurate explanation of the self-ionization of water. A water molecule can pull a hydrogen off another water molecule, which results in the two ionic species, hydroxide (OH–) and hydronium (H3O+).8
Notice that this reaction is reversible. The hydroxide can react with the hydronium ion to form two water molecules. Water is considered to be amphoteric because it can act as an acid (a molecule that produces H3O+) or a base (the produced OH– can neutralize the acid), which is the definition of amphoteric.
THE IONIC PRODUCT OF WATER
If we measure the concentration of H3O+ and OH– in pure water, they would be the same because for every H3O+ ion created, an OH– ion is created.6 This is why pure water is neutral because the concentration of the two different ions is the same. In pure water (at 25° C), the concentration is 1 X 10-7 moles/liter for both the H3O+ and the OH–. Remember that pH means the –log of the H3O+ concentration, so if you take the – log of 1 X 10-7 you get pH of 7, which we know is neutral.9
If you multiply the concentration of the H3O+ ion and the OH– ion together (i.e. 1 X 10-7multiplied by 1 X 10-7), you get 1 X 10-14. This is called the ionic product of water; it is a constant with the symbol Kw.10 Notice that if you take the –log of the Kw you get 14, which as you know is important to the pH scale.
THE PH SCALE
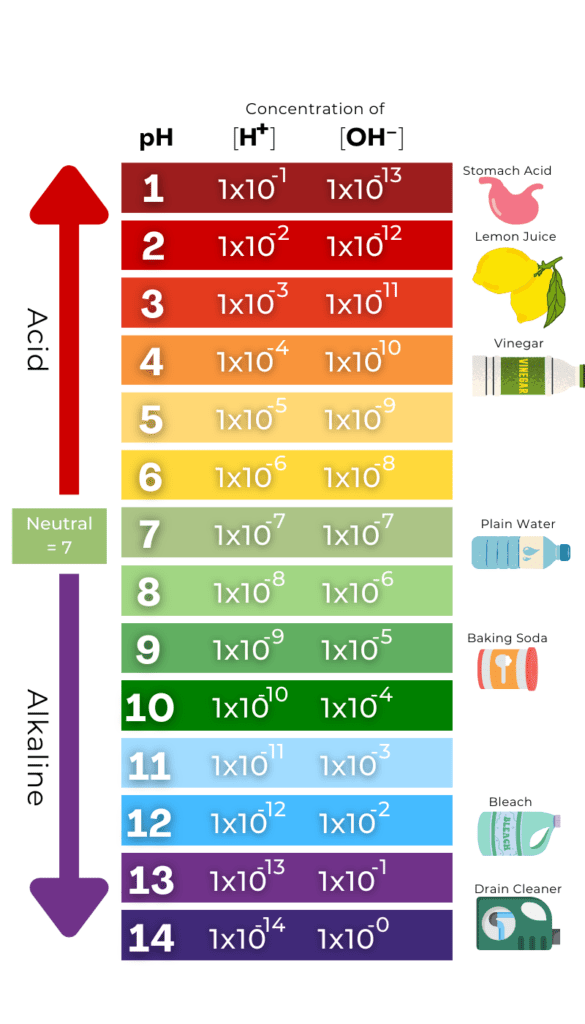

The pH scale generally goes from 0 to 14, with 7 being neutral (see figure).
This picture gives the pH, the hydronium ion concentration (symbolized by [H+], where brackets indicate concentration) the hydroxide (symbolized by [OH–]) concentration, and the pOH, which is just the negative log of the hydroxide concentration.
Notice that at pH 7, the hydronium and hydroxide ions have the same concentration. As you increase the pH, the hydronium ion concentration decreases by the same amount that the hydroxide concentration increases. This is because the Kw is always constant. You can take any pH and multiply the given concentrations of the hydronium and hydroxide ions, and you will always get 1 X 10-14.
It is also seen that a one pH increase is a 10-fold decrease in the hydronium ion concentration and increasing the pH by three results in a 1,000-fold decrease in the hydronium ion concentration.
A MORE COMPLETE DEFINITION OF PH
In reality, pH is not just simply the logarithmic concentration of the H3O+ ion, but it is actually the activity of that ion, which is not the same thing. This is expressed in the following equation:
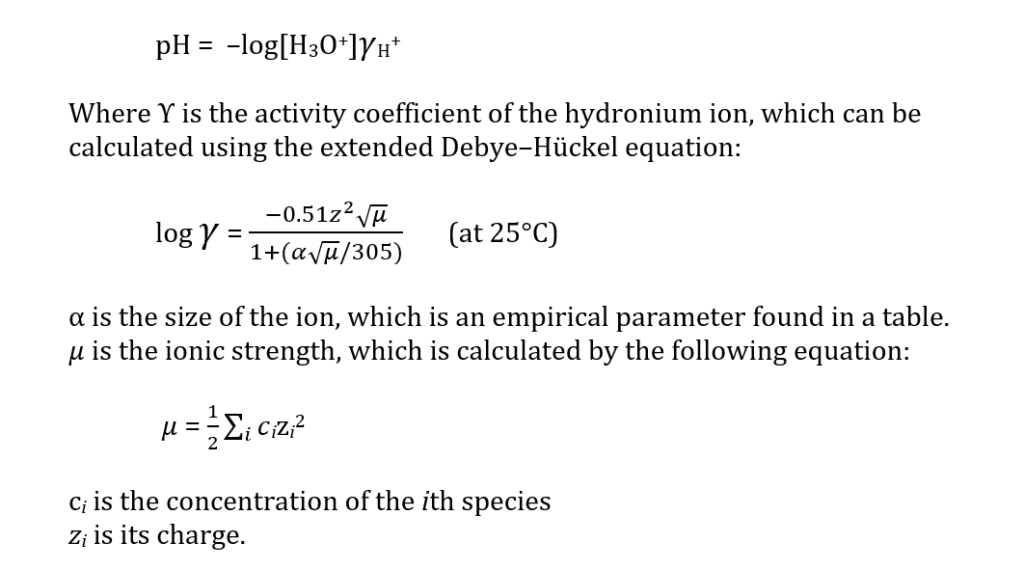

However, this only works at low ionic strengths and does not consider ion pairing. Also, the pH scale is generally recognized to go from 0 to 14, but that is just by convention. A pH of 10 has a hydronium ion concentration of 1 X 10-10 M, which is very low and difficult to conceptualize. It is much easier to just say the pH is 10. However, a pH of 0 has a 1 M hydronium ion concentration and that is pretty easy to understand.
A hydronium ion concentration of 10 M would give a pH of -1. In water, the lowest pH is about -2 and the highest pH is about 16. Remember that the H3O+ ion is the strongest acid that can exist in solution. At higher concentrations of acid or base, the solvent is no longer water, and the presence of some superacids can exist.11
DISSOLVED HYDROGEN AND PH
When talking about hydrogen, some will fail to make the important distinction as to which species of hydrogen is being discussed (see Dummies Guide to Hydrogen). The positive hydrogen ion (H+) is often referred to as “hydrogen”. But as we discussed above, this form of hydrogen is responsible for the “acid” level (pH) of water. If one assumes that the hydrogen ion is the species being discussed, they may think that adding hydrogen gas (H2) to water will change the pH of the water. But, molecular hydrogen (H2) is a neutral molecule that, when dissolved in water, has no influence on the water’s pH. Alkaline ionizers raise the pH of the water not as a direct result of adding H2 but because in order to produce H2, they must consume the H+ ions in the water, thus making the water more alkaline. Methods of producing hydrogen water such as bubbling or infusing, which simply add pure hydrogen gas to water, do so without changing the original pH of the water.
Read Alkaline, Alkalinity, & “Alkalyzed”






