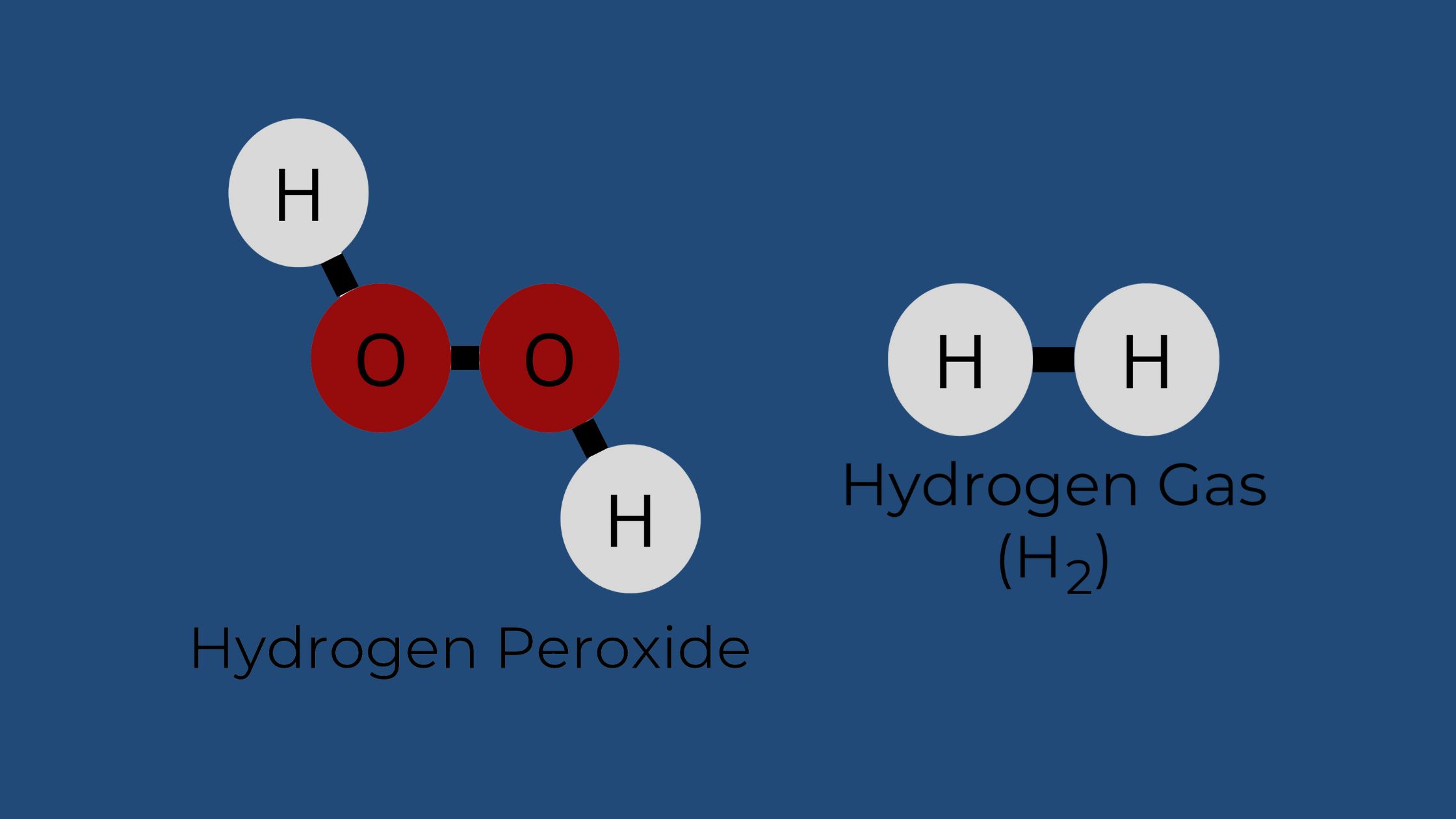
How Hydrogen Peroxide Decomposes: Water and Oxygen
Sometimes people confuse molecular hydrogen (H2) and hydrogen peroxide (H2O2), thinking they are somehow the same or can create hydrogen water. While hydrogen peroxide (H2O2) can indeed decompose, it typically separates into water and oxygen gas, not hydrogen gas. The decomposition reaction looks like this:
2H2O2 → 2H2O + O2
There isn’t any way for hydrogen to be created from hydrogen peroxide given this simple chemical equation. This is important to know as some people may believe or even teach that you can get hydrogen-rich water from hydrogen peroxide. Not only can this be potentially dangerous, but it isn’t true and you won’t get the benefits of H2-water from ingesting hydrogen peroxide.
How are molecular hydrogen and hydrogen peroxide related?
H2 gas and hydrogen peroxide both use hydrogen atoms to form their unique compounds, but as compounds, they have different chemical properties and do not exert the same therapeutic benefits. Even though hydrogen peroxide (H2O2) has the word hydrogen in its name, it doesn’t mean it is hydrogen gas. The name “hydrogen peroxide” essentially means the molecule has hydrogen atoms in its chemical makeup, and the unique bonding of two oxygen atoms is known as “peroxide” so when you combine the name, it gives you hydrogen peroxide.
How is hydrogen peroxide used? What benefits does it have?
Hydrogen peroxide (H2O2) has a variety of uses due to it being a strong oxidizing agent and disinfectant. It is often used in households as a disinfectant or surface cleaner. You can also find it in your toothpaste or laundry brightener. It is used in water to treat drinking water and wastewater to remove organic impurities and disinfect. It kills bacteria and inactivates viruses, and because it breaks down into water and oxygen, it is environmentally friendly leaving no harmful residues. In low concentrations, it’s relatively safe.
More on the safety of hydrogen peroxide
When used in high concentrations or improperly, hydrogen peroxide can be harmful. Ingesting concentrated hydrogen peroxide can be extremely dangerous and even lethal. The hydrogen peroxide commonly found in stores for household use is usually a 3%-6% solution, which is generally safe for tasks like skin cleaning and household cleaning when used correctly and in small quantities. Industrial products can contain up to 35%, but these higher concentrations can be hazardous and require careful handling and dilution.
Conclusion
In conclusion, it’s crucial to understand the differences between molecular hydrogen (H2) and hydrogen peroxide (H2O2), as well as their distinct properties. Despite some confusion, hydrogen peroxide does not yield hydrogen gas upon decomposition but instead forms water and oxygen gas. This distinction is particularly important to note, especially when considering claims about creating “hydrogen-rich water” from hydrogen peroxide. Such claims are not only false but can also be hazardous.
Read How to Get Molecular Hydrogen
Read The Complete Guide to Molecular Hydrogen
Read Dummies Guide to Hydrogen