HALF-REACTIONS
As explained previously, ORP is measured in volts and informs us of a solution’s oxidizing or reducing potential.1 An ORP probe measures the voltage difference between redox couples in accordance with their half reactions2 (see table 17.1 below).
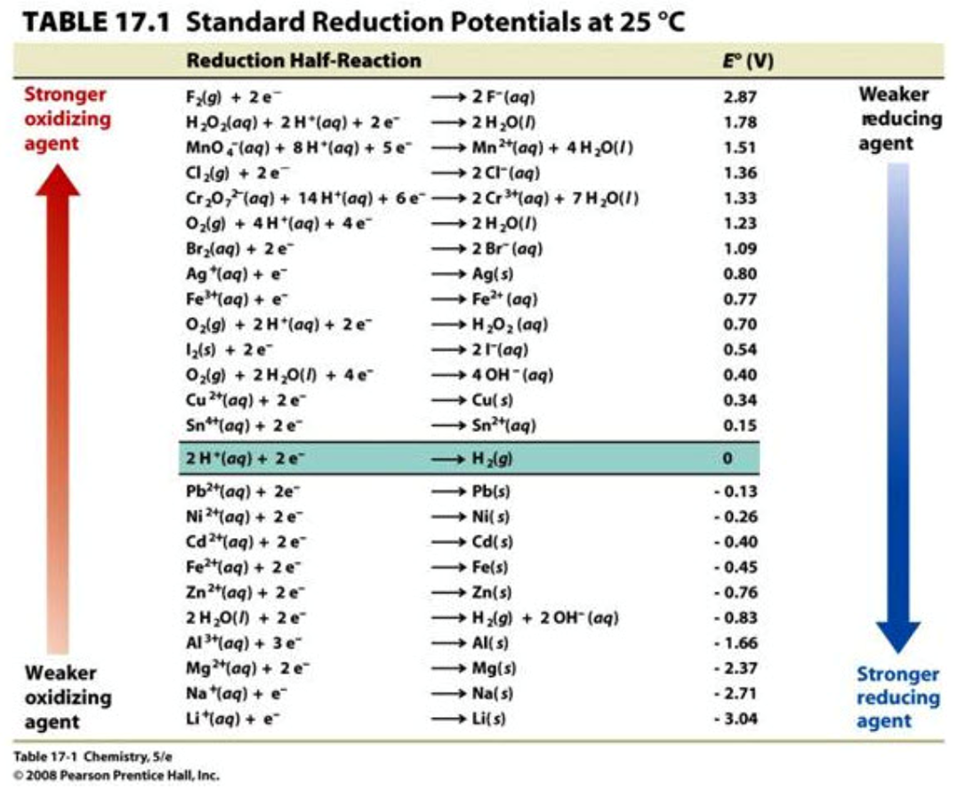
For example, the first redox couple fluorine (F2) is a strong oxidizing agent. If you measured the voltage difference between F2 and its reduced species fluoride (both at a 1 Molar concentration), you would get a voltage reading of 2.87 V.
Notice that the reduction potential of hydrogen: 2H+ + 2e– => H2(g) is zero. This is because all redox values are based off of the standard reduction potential for hydrogen, which has been defined as zero.3 Just as sea level is defined as zero elevation or water freezes at 0°C, the voltage produced by 1 M H+ (pH 0) to H2 (g) (pressure equals 1 bar) is defined as zero.3It’s actually estimated to be 4.44 ± 0.02 V at 25 °C,4 but we define it as zero at all temperatures.3 This allows us to make comparisons; for example, the elevation of a mountain, the boiling point of alcohol, or the ORP of a redox couple. All redox reactions are compared to the Standard Hydrogen Electrode (SHE).2
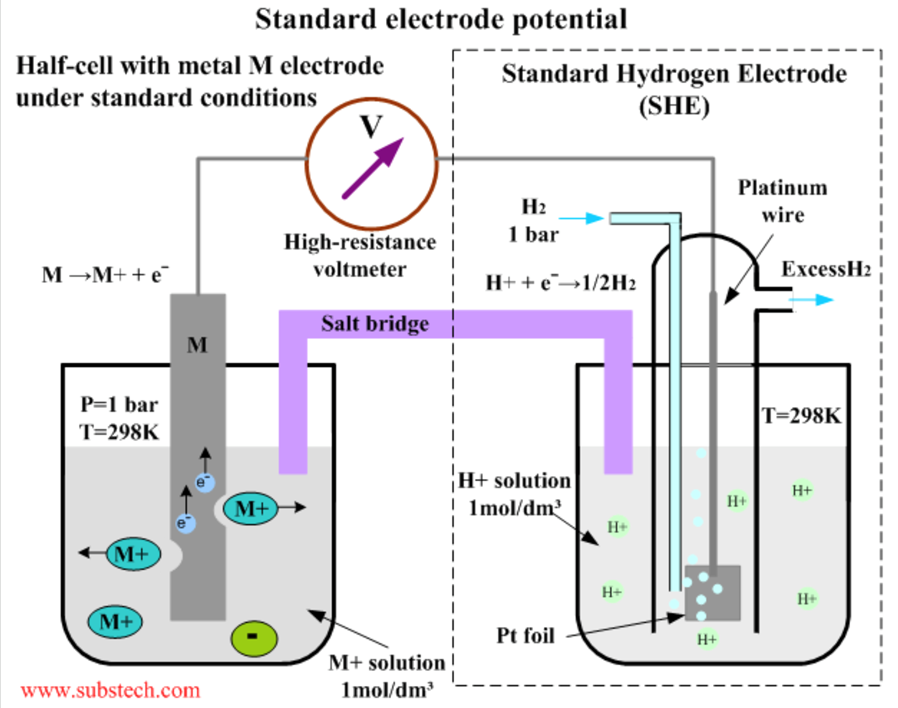
This diagram shows how the standard potential Eoof a species (M) can be determined. The M electrode contains M ionic species in equilibrium with the non-ionic M species. The potential is referenced to the standard hydrogen electrode on the right.
ORP METERS
The ORP meter is generally a two-electrode system (some may have a third as a counter -electrode). One is the platinum electrode called the working electrode where the oxidation-reduction reactions occur. It either serves as an electron donor or an electron acceptor, depending upon the test solution. The other is a reference electrode (usually Ag/AgCl), which is calibrated back to the standard hydrogen electrode. The reference electrode is filled with a saturated solution (3 M) of KCl. This two electrode system makes a potentiometric measurement measured in volts.2
NERNST EQUATION
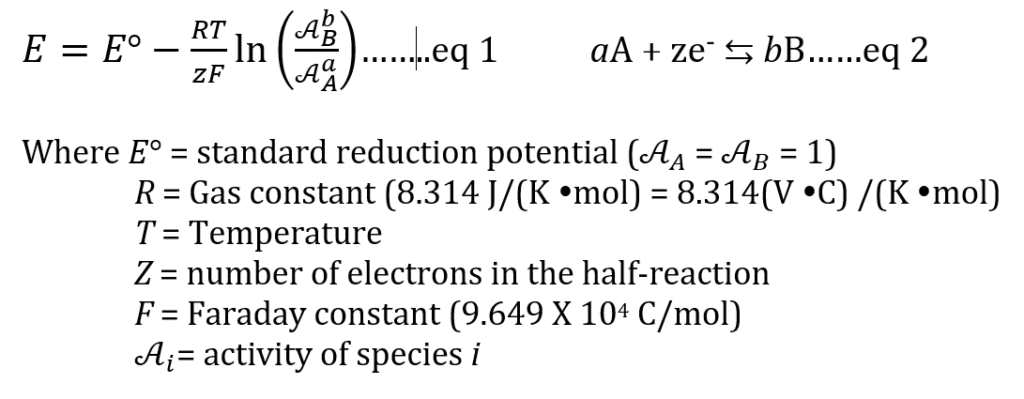
Under theoretical conditions, the ORP can be predicted by using the Nernst Equation (eq 1) for the half reaction (eq 2)
Notice that if the redox couple H2 (g) and H+ ions are entered into the Nerst equation at standard conditions then ln (1) equals zero, which causes the E to be zero because Eo is zero.
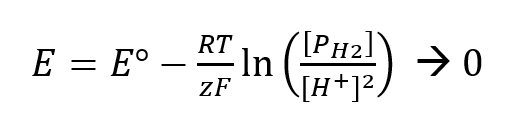
ORP meters are not very common amongst those in the chemistry field, because it only measures the potential of the solution and not a specific ion or molecule. This is especially true with solutions that have low conductivity and when the concentrations of the redox couples are low.
This is the case when H2 and H+ ions are the redox couples, as these concentrations are relatively small on the micro to nanoscale. If molecular hydrogen is the chemical species of interest, then the preferred method for testing is to simply measure the concentration of molecular hydrogen.


