Introduction
It’s almost impossible to have a conversation about ionized water without hearing the terms alkaline, alkalinity, or alkalized (also misspelled as “alkalyzed”). Distributors/marketers and sales agents will frequently use the phrase “the alkalinity of the water” when what they are really referring to is its higher pH.
The Root of the Confusion
One cannot look at the terms alkaline, alkalinity, and alkalized without noticing that they all contain the common root term alkali. Because of this, it is easy to assume that all three derivatives refer to the same property of ionized water. In order to clear up the confusion, it is necessary to examine these terms in more detail.
Etymology of the Root Term
The term alkali has its origin in 14th century Arabic and is a compound term (al + qili) meaning the calcined (or “roasted”) ashes. The ash, which remained after burning a plant such as saltwort, would be mixed with water to form a mildly basic solution. These solutions could then be combined with animal fats to produce soap, a process we now refer to as saponification. It was not until the 19th century, when early chemists began to identify and categorize elements, that the ”alkalis” were organized and placed together in a meaningful way according to their chemical properties.
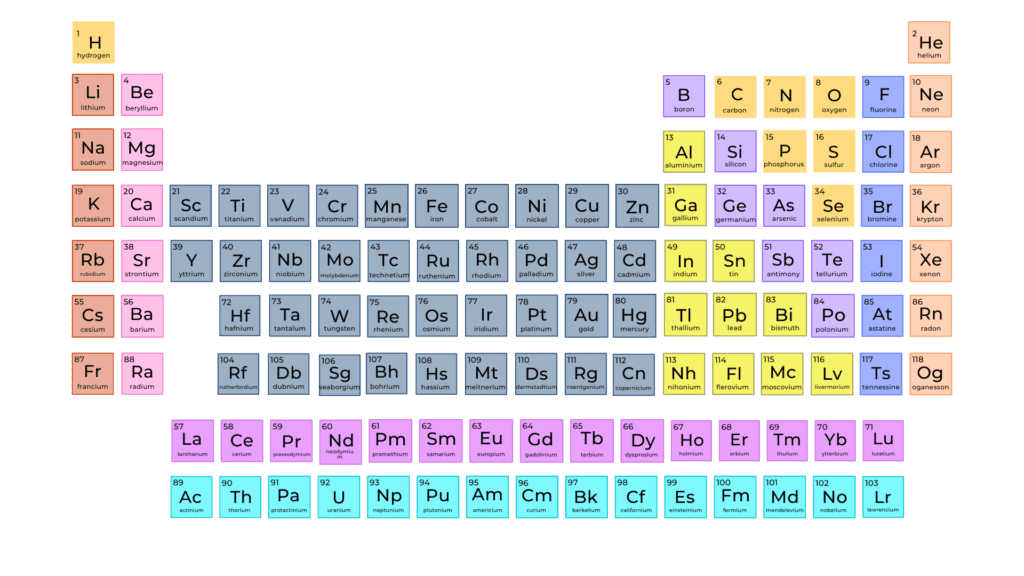
The Periodic Table
Most people who have taken a high school chemistry class will remember the Periodic Table of the Elements, which contains the known elements of the universe arranged according to their atomic structure. Columns one and two, the alkali metals and alkaline earth metals, contain 12 elements including the familiar ones, sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca).
Characteristics of Alkalis
When these metals are added to water in their pure, elemental (non-ionic) form, they readily react with water to produce hydrogen gas and hydroxide ions, which increases the pH, making the water alkaline. The ability of alkali metals to raise the pH when dissolved in water helps explain how the term “alkaline” has come to describe water whose pH is greater than 7.
An alkaline solution simply means it has a pH greater than 7. Alkalinity, on the other hand, refers to a solution’s quantitative ability to neutralize an acid. Therefore, you can have water with high (alkaline) pH and low alkalinity, or high alkalinity and low pH.
Read Understanding pH
Ionized Water and Alkalinity
When discussing the alkalinity of ionized water (the ability to neutralize acid), it is important to remember that the alkalinity of ionized water is essentially established by the alkalinity of the tap water from which it is produced (although a slight increase in acid-neutralizing capacity is realized due to the increase in hydroxide levels). This can easily be verified by using standard test strips to compare the alkalinity of the ionized water with the tap water. The reason that the tap and ionized water have similar alkalinity is that a water ionizer does not add “buffering compounds” to the water during electrolysis. The alkalinity of municipal water systems varies throughout the world and is controlled by a number of factors. These include such things as exposure to rocks and soils, salts, certain plant activities or industrial wastewater discharges (detergents and soap-based products are alkaline). If an area’s geology contains large quantities of calcium carbonate (limestone), source water will tend to have carbonates in the water, which increase alkalinity. Alkaline ionized water can therefore have either relatively high or low alkalinity, depending upon the amount of buffering compounds contained in the water, but its alkalinity will be about the same as the source water to which the ionizer is connected.
Ionized water sales reps often perform a demonstration (“soda demo”) which provides first-hand evidence for the low alkalinity of ionized water. During this demonstration, a small amount of carbonated soda (acid pH) is poured into a relatively large volume of alkaline water (usually a few liters) containing pH drops, and the pH of the entire container of alkaline water immediately becomes acidic as evidenced by the change in color with the pH drops. This is a real-life demonstration of alkaline water’s inability to buffer acid and therefore its low alkalinity.
In fact, from a chemical stoichiometric perspective, 1 teaspoon of baking soda can neutralize the same amount of acid as 600 liters of alkaline water at a pH of 10. Viewed in another way, it would take 10,000 liters of pH 10 water to neutralize just 1 liter of stomach acid at a pH of zero. This demonstrates alkaline water’s low alkalinity and its inability to buffer or neutralize acid.
Chemical Buffers
The definition of the term alkalinity introduces a new word to our vocabulary, buffer. To better understand alkalinity, we will examine the concept of a buffer. A buffer is a substance that gives water the ability to resist changes in its pH when small amounts of acids or bases are added to it. A buffer is composed of two compounds, a weak acid and its conjugate base. A good example of this is the alkaline buffering system utilized by our body to maintain an alkaline blood pH. This system utilizes carbonic acid (H2CO3, weak acid) and bicarbonate (HCO3–, conjugate base) to maintain the blood’s pH in the very narrow range of about 7.3 – 7.4. The maintenance of this narrow pH range is critical to the chemical reactions that occur within the blood and cells.
Is Ionized Water Alkaline, Alkalized (or Both?)
Ionized water undergoes a process called electrolysis during which two separate reactions, oxidation (at the anode) and reduction (at the cathode) occur in two separate chambers separated by a membrane. This results in two separate streams of water, an acidic stream (low pH) and an alkaline stream (high pH). The definition of “alkaline” is simple-having a pH greater than 7. Therefore, it is correct to call water whose pH is greater than 7 “alkaline”, as in “alkaline water”.
The adjective alkalized is another term often used in the ionized water community to describe ionized water. In chemistry, this term is a fairly simple one to define- it simply means that the pH of the solution has been increased. Therefore, any water whose pH has been raised into the alkaline range (above 7) would qualify as being “alkalized”. From this explanation, it can be seen that a “water ionizer” that increases the pH of the tap water can be called an “alkalizer” of tap water and that after processing, the resultant water can be considered “alkalized”.
Some have incorrectly suggested that there is a difference between water that was “alkalized” via a water ionizer and water “alkalized” via the addition of mineral compounds in combination with or completely without the use of electrolysis. In fact, some have even gone so far as to claim that if the water was made alkaline by a process other than “electrolysis”, then it is not considered “alkalized” and that the pH was “chemically induced” and thus harmful, as opposed to “electrically induced” and thus beneficial. However, this is simply inaccurate. The only difference is that not every method of making an alkaline pH produces hydrogen gas. Nevertheless, the alkaline properties of the waters are, by definition, identical (i.e. more OH– ions and fewer H+ ions).
“Adding Minerals”
It is true that some ionizer manufacturers have remineralization filters that add mineral electrolytes or ions such as sodium (Na+), magnesium (Mg++), potassium (K+), calcium (Ca++), etc. However, the addition of these minerals alone does not increase the pH, but simply improves the conductivity (ability of water to conduct electricity) of water, thereby improving the efficiency of the overall electrolysis process. These remineralization filters are often used in water ionizer installations where the source water is either reverse osmosis (RO), or simply lacking adequate mineral levels necessary for electrolysis. Water ionizer filters may contain calcium sulfite (CaSO3), which also increases the conductivity of the water as it dissociates into its ions, Ca++ and SO3–. Some units also have an external “port” which provides for the addition of electrolytes by adding a compound such as calcium glycerophosphate to the water prior to electrolysis. Devices that utilize these and other methods for increasing electrolyte levels may realize an improvement in electrolysis performance resulting in an enhanced level of H2 gas production.
What about the non-electric type of ionizer unit?
In addition to the electric water ionizer most commonly seen for producing alkaline water, there are “non-electric” units and tablets that can also produce alkaline water. These units can produce alkaline water and dissolved molecular hydrogen in the water not through electrolysis, but by combining water with elemental magnesium (Mg + 2H2O ==> Mg(OH)2 + H2). As seen in the equation, the reaction results in the production of hydrogen gas (H2) and magnesium hydroxide (Mg(OH)2), which is responsible for the increase in pH. Because this technology does not use electricity/electrolysis to produce alkaline water, some will say that this is an “inferior” way of producing alkaline hydrogen water. But, there is no reason to question either the quality of the drinking water or the dissolved hydrogen produced. As far as the higher pH, the end result of an increased level of hydroxide ions is identical to that produced during electrolysis. Indeed, the process of adding electrons to the water via either electrolysis or metallic magnesium is the exact same thing (2e– + 2H2O –> H2 +2OH–). In order to maintain a final pH closer to neutral, most tablets manufactured today include some form of acid to neutralize the hydroxide produced.