I. Introduction to Hydration and Its Importance
Water, the universal solvent, is fundamental to life, playing a pivotal role in every biological process within our bodies [1]. Its unique properties make it indispensable for cellular function, biochemical reactions, and physiological processes [2]. Despite its ubiquity, the importance of proper hydration is often underestimated or misunderstood. This article aims to provide a comprehensive, scientifically grounded overview of hydration, addressing its fundamental principles, physiological impacts, and common misconceptions. We’ll explore the latest scientific understanding of hydration, from molecular interactions to systemic effects, and touch upon emerging research in molecular hydrogen as it relates to optimizing hydration.
II. Fundamentals of Water and Hydration
Water’s molecular structure, consisting of two hydrogen atoms covalently bonded to an oxygen atom at an angle of 104.45°, gives rise to its exceptional properties [3], as shown in Figure 1. This bent shape creates a polar distribution of charge, with a slightly negative charge near the oxygen atom and slightly positive charges near the hydrogen atoms. This polarity enables hydrogen bonding (shown in Figure 2) between water molecules, a key factor in water’s unique properties [4].
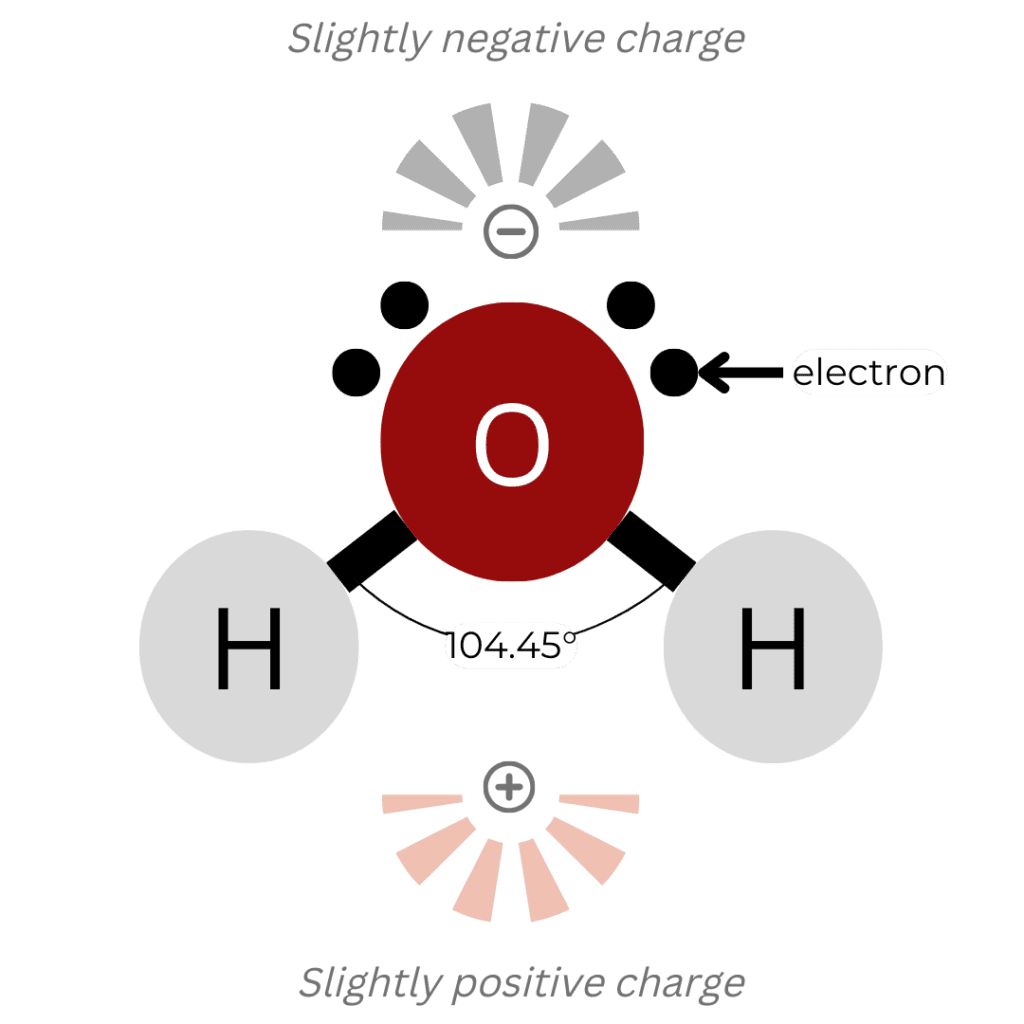
Figure 1. A water molecule consists of two hydrogen atoms and one oxygen atom with a bond angle of 104.45 degrees which gives it unique polarity.
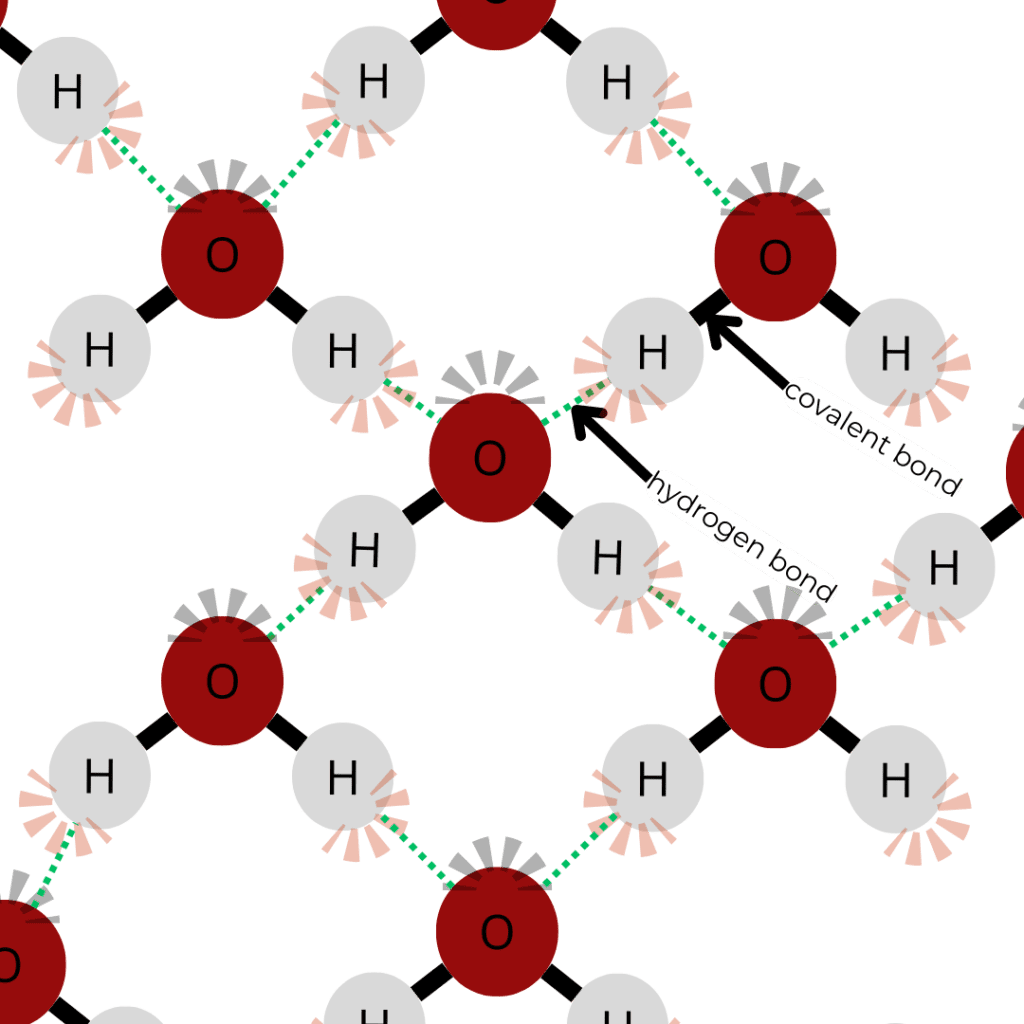
Figure 2. A 2–D depiction of water molecules forming hydrogen bonds, where the slightly positive hydrogen loosely bonds with the slightly negative oxygen.
The hydrogen bonds in water are responsible for several critical characteristics:
- High boiling point: The energy required to break these bonds results in water’s high boiling point of 100°C at standard pressure [5].
- High specific heat capacity: Water can absorb or release substantial amounts of heat with minimal temperature change, crucial for thermal regulation in biological systems [6].
- High surface tension: This property is essential for capillary action in plants and the formation of cell membranes [7].
- Universal solvent capabilities: Water’s polarity allows it to dissolve a wide range of substances, facilitating biochemical reactions and transport of nutrients [8].
In living systems, water serves multiple essential functions:
- Solvent and medium for biochemical reactions: Most cellular processes occur in aqueous solutions [9].
- Transport of nutrients and waste products: Blood plasma, lymph, and cellular fluids are primarily water [10].
- Structural component: Water contributes to the three-dimensional structure of biomolecules like proteins and nucleic acids [11].
- Thermoregulation: Water’s high specific heat and evaporative cooling properties are crucial for maintaining body temperature [12].
- Lubrication: Water is a key component of synovial fluid in joints and mucus in various organs [13].
The polarity of water molecules facilitates the formation of hydrophilic and hydrophobic interactions, which are fundamental to the structure and function of proteins, cell membranes, and other biological structures [14]. These essential interactions allow for protein folding, enzyme-substrate binding, and the assembly of biological membranes [15].
III. Hydration in the Human Body
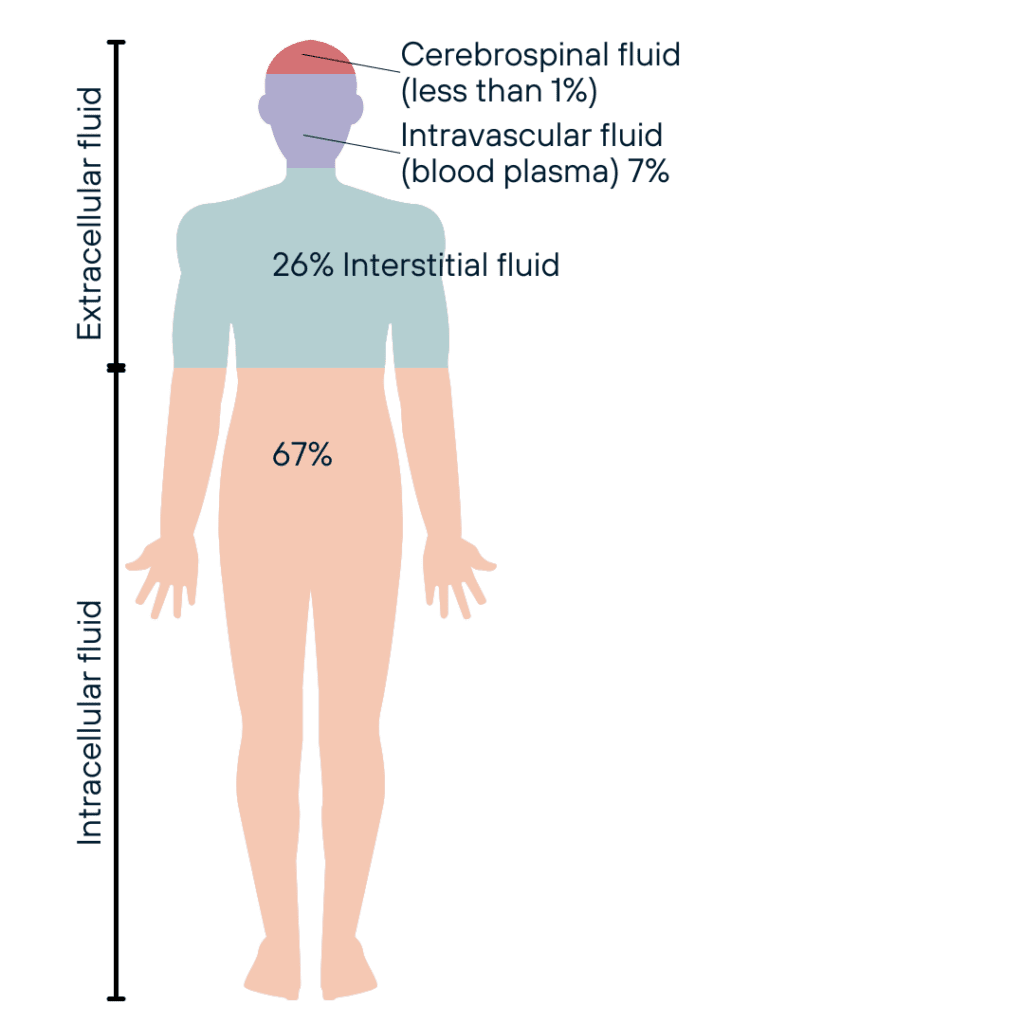
The average adult body is 55% to 75% water, with variations depending on age, sex, and body composition [16]. This water is distributed across various compartments:
- Intracellular fluid (ICF): ~40% of body weight; 67% of the fluid in the body
- Extracellular fluid (ECF): ~20% of body weight; 33% of the fluid in the body
- Interstitial fluid: ~15% of body weight; 26% of the fluid in the body
- Plasma: ~5% of body weight; 7% of the fluid in the body
- Transcellular fluid (e.g., cerebrospinal fluid, synovial fluid): <1% of body weight; <1% of the fluid in the body [17]
Water plays a vital role in numerous physiological processes:
- Cardiovascular system: Water is the primary component of blood plasma, crucial for maintaining blood volume and pressure. It also plays a role in the regulation of electrolyte balance [18].
- Respiratory system: Water humidifies inhaled air and is essential for the maintenance of mucous membrane integrity. It’s also involved in the formation of surfactants in the lungs [19].
- Digestive system: Water is a major component of digestive secretions and is crucial for nutrient absorption. It aids in the breakdown of food particles and facilitates the movement of material through the gastrointestinal tract [20].
- Urinary system: Water is essential for the filtration of blood in the kidneys and the excretion of waste products. It also helps maintain the proper concentration of electrolytes [21].
- Musculoskeletal system: Water contributes to the lubrication of joints through synovial fluid and helps maintain muscle tone through proper hydration of muscle fibers [22].
- Nervous system: Water is a major component of cerebrospinal fluid, which provides mechanical protection to the brain and spinal cord. It’s also crucial for the maintenance of proper electrolyte balance necessary for nerve signal transmission [23].
- Integumentary system: Proper hydration is essential for maintaining skin elasticity and facilitating thermoregulation through sweating [24].
At the cellular level, proper hydration is crucial for maintaining cell volume, which in turn affects various metabolic processes [25]. Changes in cell volume can influence protein synthesis, cell proliferation, and even gene expression [26]. The movement of water across cell membranes is facilitated by specialized proteins called aquaporins, which play a fundamental role in cellular hydration and overall fluid balance in the body [27].
Osmotic gradients, created by differences in solute concentrations across membranes, drive water movement between fluid compartments. This process is critical for maintaining proper cellular function and overall fluid balance [28].
IV. Optimal Hydration and Health
Maintaining optimal hydration is a delicate balance influenced by numerous factors including climate, physical activity, diet, and overall health status [29]. Dehydration, even mild, can lead to significant physiological and cognitive impairments [30].
Effects of dehydration include:
- Decreased cognitive function: Impaired concentration, short-term memory, and decision-making [31].
- Reduced physical performance: Decreased endurance, increased perceived exertion, and impaired thermoregulation [32].
- Increased risk of urinary tract infections and kidney stones [33].
- Alterations in mood and increased fatigue [34].
- Impaired cardiovascular function: Increased heart rate and decreased stroke volume [35].
Symptoms of dehydration include thirst, dark urine, dry mouth, fatigue, dizziness, and in more severe cases, rapid heartbeat and decreased skin turgor [36].
Conversely, overhydration, though less common, can also be dangerous. Excessive water intake can lead to hyponatremia, a condition where sodium levels in the blood become diluted [37]. Symptoms of hyponatremia include nausea, headaches, confusion, and in severe cases, seizures or coma [38]. This condition is more common in endurance athletes who consume large volumes of water without adequate electrolyte replacement [39].
The often-cited recommendation of “eight 8-ounce glasses of water per day” lacks scientific basis and may not be suitable for everyone [40]. Hydration needs vary based on factors such as body size, activity level, climate, and diet. The Institute of Medicine suggests a more nuanced approach, recommending a total daily water intake (including from foods) of about 3.7 liters for men and 2.7 liters for women, with adjustments for individual circumstances [41].
Practical strategies for maintaining proper hydration include:
- Drinking water regularly throughout the day
- Increasing fluid intake during physical activity or in hot environments
- Consuming water-rich foods like fruits and vegetables
- Monitoring urine color (pale yellow indicates good hydration)
- Drinking water before, during, and after exercise [42]
Recent research has explored more sophisticated methods of assessing hydration status, including bioimpedance analysis, urine specific gravity, and plasma osmolality measurements [43].
V. Common Myths and Misconceptions
Several myths persist regarding hydration, often propagated by marketing claims or misinterpretation of scientific data. Some of these myths include:
- “Structured water” or “hexagonal water” provides superior hydration: There’s no scientific evidence supporting these claims. All water, regardless of its source, becomes structured in the same way when it enters the body [44].
- Microclustering: in the context of water, refers to the concept that water molecules can be arranged in smaller, more stable clusters compared to conventional water. Proponents of microclustering claim that these smaller clusters allow water to be better absorbed by cells and tissues, potentially enhancing hydration and nutrient absorption. However, again, the scientific community rejects claims of microclustering because it has been extensively studied and refuted. Read Microclustering: The making of a myth part 1, part 2, part 3, and part 4.
- Distilled water is harmful or “dead” water: While distilled water lacks minerals, it’s not harmful when consumed as part of a balanced diet that provides necessary minerals. The body doesn’t rely on water as a primary source of minerals [45].
- Caffeinated beverages are dehydrating: While caffeine has a mild diuretic effect, caffeinated beverages contribute to overall fluid intake and do not lead to dehydration when consumed in moderate amounts [46].
- Sports drinks are always better than water for hydration: For most people engaged in moderate exercise, water is sufficient. Sports drinks are beneficial primarily during prolonged, intense exercise or in very hot conditions where electrolyte replacement is necessary [47].
- You can never drink too much water: Excessive water intake can lead to hyponatremia, particularly in endurance athletes or individuals with certain medical conditions [48].
- These myths, and others, continue to saturate the market, however, having an understanding of the scientific principles of molecular biology and physiology will allow us to discern what is logical and illogical. In summary, many common beliefs about hydration lack scientific backing and can be misleading. Understanding these myths helps promote informed decisions about proper hydration practices.
VI. Molecular Hydrogen and Hydration
Recent research has explored the potential benefits of molecular hydrogen (H2) in optimizing hydration and overall health. Molecular hydrogen is a gas that can dissolve in water, creating hydrogen-rich water [49, 50].
Some studies suggest that H2-rich water may:
- Enhance cellular hydration by improving the function of aquaporins [51].
- Provide antioxidant effects by selectively reducing harmful reactive oxygen species [52, 53].
- Improve exercise-induced fatigue and reduce lactate levels [54].
- Potentially enhance cognitive performance and mood [55].
Perhaps some of these benefits are attributed to some indirect improvements in cellular hydration from molecular hydrogen. For example, combating oxidative stress, inflammation, and cellular damage would allow the cell to function properly so that it can maintain optimal hydration, which is essential for cellular function. Nevertheless, human studies are needed to further evaluate the impact hydrogen on hydration.
However, the idea that hydrogen somehow H2 somehow makes water more bioavailable, effectively circumventing the known mechanisms of osmolarity is inaccurate. Additionally, some may claim that when hydrogen neutralizes hydroxyl radicals and forms water (H2 + 2·OH →2H2O) that this leads to increased hydration. However, although water molecules may be produced by this reaction, the volume of water produced would be extremely low, less than 0.01 mL. In summary, although H2 may indirectly optimize cellular hydration due to some of the indirect mechanisms, there is no evidence that H2 directly increases cellular hydration.
VII. Conclusion
Proper hydration is fundamental to human health and well-being, influencing every aspect of physiology from the molecular to the systemic level [55]. Understanding the science behind hydration empowers individuals to make informed decisions about their fluid intake and overall health. As research continues to evolve, particularly in areas like molecular hydrogen and advanced hydration assessment techniques, our understanding of optimal hydration strategies may be refined. Nonetheless, the importance of maintaining adequate hydration through regular water consumption remains a cornerstone of good health.