HYDROXIDE (OH–) CONFUSION

There appears to be a lot of confusion about the hydroxide ion, i.e. OH–, (also incorrectly called just “hydroxyl”, which ironically is a free radical or even “hydroxyl water”) within the “ionized water community”. This is because numerous websites, marketers, sales pitches, explanations, etc. claim that hydroxides are antioxidants. 1,2
Recently, however, there are other voices purporting that this hydroxide is not an antioxidant, but the polar opposite: a free radical.3 The reality of all this confusion is that it is neither.
NOTION AS AN ANTIOXIDANT
It seems that most people teach that hydroxide is an antioxidant. This false teaching probably stems from the following four perspectives:
- The wide held belief in the fallacy that antioxidants are negative whereas free radicals are positive,4 coupled with the fact that hydroxide (OH–) has a negative charge.
- Alkaline ionized water has a high pH and thus contains more hydroxides (OH–) ions, which are negatively charged
- Alkaline ionized water does exhibit a negative oxidation-reduction potential (ORP) and has antioxidant activity.5
- The fact that molecular hydrogen (H2) was not recognized as the reason for the –ORP, the antioxidant activity6, and the therapeutic effects until about 2007,7 which means ionized water was marketed decades before the proof of H2 was established. 8
The belief that hydroxide, or even just water itself, is the antioxidant has been around a long time with comparisons of molecular weight as shown.9 This of course is considered pseudoscience, as there is no truth to it.
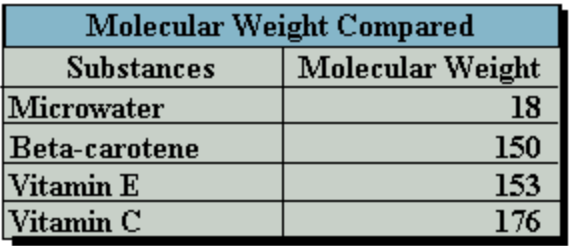
The figure to the left is commonly used to promote the false idea that the water itself is the antioxidant. However, as mentioned, the real antioxidant is H2, which for comparison has a molecular weight of only two grams per mole10.
NOTION AS A FREE RADICAL
It has also been taught that hydroxide (OH–) is a free radical. I am not sure how this came to be, but perhaps it was because of the similarity between the terms hydroxide (OH–) and hydroxyl OH. ). The latter is actually one of the the most reactive oxygen radicals there are.11 Notice the black dot on the upper right side of the H in the symbol (OH.); this is indicative of an unpaired electron, which means it is a free radical.10
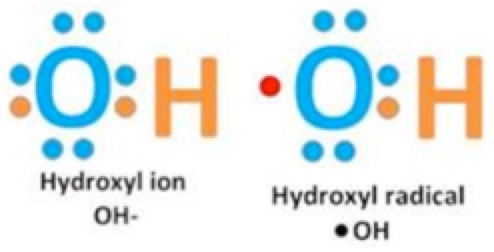
Those who are not familiar with chemistry often use hydroxyl and hydroxide interchangeably—even though they are entirely different species. The hydroxide ion (far left) is not radically reactive at all, as it contains stable paired electrons.
HYDROXIDE IS A BASE
Hydroxide (OH–) or the hydroxyl ion is a component of water. Water dissociates to form OH– ions and H3O+ ions.10 That is: 2H2O => OH– and H3O+(learn more here).
Notice that this reaction is reversible, which means that the hydroxide ion can react with the hydronium ion (H3O+) to form two water molecules. So yes, hydroxides are reactive when discussing Acid/Base chemistry,10 but not as a biological antioxidant.
ANTIOXIDANT IN IONIZED WATER IS MOLECULAR HYDROGEN
Another thing to consider is what would happen if hydroxide (OH–) acted as an antioxidant and donated an electron? It would turn into the most cytotoxic oxygen radical: the hydroxyl radical (OH.). Thus the reaction produced a radical more reactive than the first one, which isn’t going to happen. Because it is clear that hydroxide is not an antioxidant, much less a free radical, touting it as either one is another reason why many ridicule the concept of ionized water.
The take-home message is that hydroxide (OH–) is higher in alkaline water according to the definition of pH, but it is not a biological antioxidant. The therapeutic agent in ionized water is clearly dissolved molecular hydrogen gas (H2).7,8